Electrofermentation: Steering microbial metabolism with electricity
Simply put, fermentation is a natural process where microorganisms like bacteria or yeast break down sugars (or other organic compounds) without oxygen to gain energy. As they do so, they also produce useful byproducts such as alcohol, lactic acid, or short-chain fatty acids like acetate, butyrate, and propionate, depending on the microbes involved and the surrounding conditions. Humans have harnessed this process for thousands of years to make bread, yogurt, and wine.
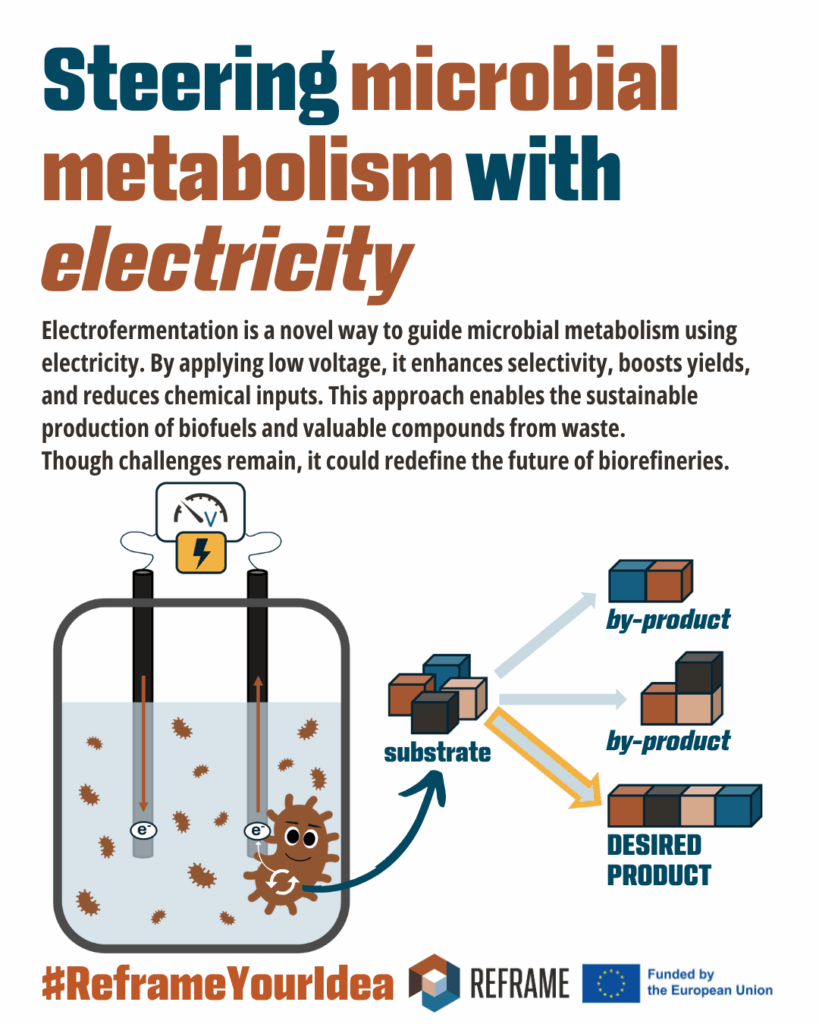
Today, fermentation is at the core of industrial biotechnology: we use it in bioreactors to produce biofuels, bioplastics, and valuable chemicals, even starting from organic waste. But, what happens if you connect an electrode to a bioreactor?
That’s where Electrofermentation (EF) comes in a cutting-edge technology that combines electricity with microbial metabolism.
EF uses a small voltage to guide how microbes behave and what they produce. Think of it like giving electrons a gentle push, from point A to point B, which allows a current to flow and creates a new set of conditions inside the bioreactor. This push can shift the balance of microbial reactions, steering them toward more valuable products.
What does this voltage actually do?
When you apply a low voltage, EF can:
- Modulate redox conditions: that is, influence the environment that controls how electrons are transferred in chemical reactions. These redox conditions are like a traffic system for electrons: they affect the direction, speed, and balance of oxidation-reduction reactions inside the reactor.
- Enhance product selectivity: e.g., favoring short-chain fatty acids or specific biofuels.
- Increase carbon fixation: helping convert CO₂ or organic waste into new carbon-based products.
So how does it work?
EF relies on a close interaction between microbes and electrodes, either directly or through molecules that shuttle electrons (mediated transfer). This microbial-electrode interface can redirect metabolic pathways, favoring more oxidized or reduced compounds, depending on the voltage applied.
Why is Electrofermentation important?
EF sits at the crossroads of bioelectrochemistry, microbial ecology, and reactor engineering. It opens a new frontier in biotechnology, especially for turning organic waste into high-value compounds in a more selective, flexible, and sustainable way.
It’s a promising technology, but challenges remain:
- We still lack a full mechanistic understanding of how it works at the microbial and molecular level.
- Reactor design is complex, from how electrons reach the microbes, to how gases and nutrients move.
- Scaling up while keeping the energy input efficient is an ongoing hurdle.
The question now isn’t whether EF works, but how far it can go. It might become a core enabler of the next-generation biorefinery, especially where waste variability, selectivity, and process flexibility matter most.
Sources:
- Bhagchandanii, D. D., Babu, R. P., Sonawane, J. M., Khanna, N., Pandit, S., Jadhav, D. A., Khilari, S., & Prasad, R. (2020). A Comprehensive Understanding of Electro-Fermentation. Fermentation, 6(3), 92.
- Yunkai Tan, Yihang Xiao, Tianwei Hao, Carbon fixation via volatile fatty acids recovery from sewage sludge through electrochemical–pretreatment–based anaerobic digestion, Water Research, Volume 258, 2024
- Ki D, Parameswaran P, Popat SC, Rittmann BE, Torres CI. Effects of pre-fermentation and pulsed-electric-field treatment of primary sludge in microbial electrochemical cells. Bioresour Technol. 2015 Nov;195:83-8.
- Pilania, P., Bhushan, K., & Phutela, U. G. (2025). Electro-Fermentation for Biofuel and Biochemical Production. Fermentation, 11(4), 219.
- Andrea Schievano, Tommy Pepé Sciarria, Karolien Vanbroekhoven, Heleen De Wever, Sebastià Puig, Stephen J. Andersen, Korneel Rabaey, Deepak Pant,Electro-Fermentation – Merging Electrochemistry with Fermentation in Industrial Applications,Trends in Biotechnology,Volume 34, Issue 11,2016.
- Bernardino Virdis, Robert D. Hoelzle, Angela Marchetti, Santiago T. Boto, Miriam A. Rosenbaum, Ramiro Blasco-Gómez, Sebastià Puig, Stefano Freguia, Marianna Villano, Electro-fermentation: Sustainable bioproductions steered by electricity,Biotechnology Advances,Volume 59, 2022.
#ReframeYourIdea #Electrofermentation #ScienceForEveryone